

The French National Research Network on Kidney Cancer—UroCCR—is a multidisciplinary medico-scientific network focused on therapeutic management and applied research in the field of kidney cancer.
It includes 60 centres across France and abroad, involving university hospitals, regional hospitals, private clinics, and cancer research centres.
These data warehouses contain information on more than 21,000 patients, representing over 10 million real-world data records (demographic and clinical data, data associated with biological samples, PREMs and PROMs, experimental results).
They also include a virtual bio-collection of biological samples. In addition, the BRCs of the participating centres are certified by IBiSA, recognising their high level of quality.
Furthermore, close collaboration with the CARARE (CAncer RAre du REin) network enables joint efforts to improve knowledge about rare kidney tumours and offer better care at the national level.
UroCCR is currently included in the list of databases and real-world studies that have been identified and may be used to respond to requests for additional data from the French National Authority for Health for the evaluation of health products and technologies.
The UroCCR Network is recognised in both academic and industrial research circles, primarily due to its labeling as a clinical investigation network by the ANR (French National Research Agency). This status has allowed the Network to establish a support desk—UroDM—within its governance structure to foster research projects focused on medical devices. Furthermore, the Network utilises the UroCONNECT tool, developed in partnership with the company Résilience, which digitises nursing coordination for perioperative renal surgery pathways in enhanced rehabilitation and outpatient care.
To date, more than 250 ancillary projects have been submitted and validated by the Scientific & Ethics Committee of the UroCCR Network.
The Network is committed to collaborative working: it comprises more than 750 professionals in five different specialities (urological surgeons, oncologists, radiologists, biologists and anatomic pathologists), as well as translational researchers, learned societies such as the French Association of Urology, the French Network of Cancer Registries (FRANCIM) and, of course, the ARTuR patient association.
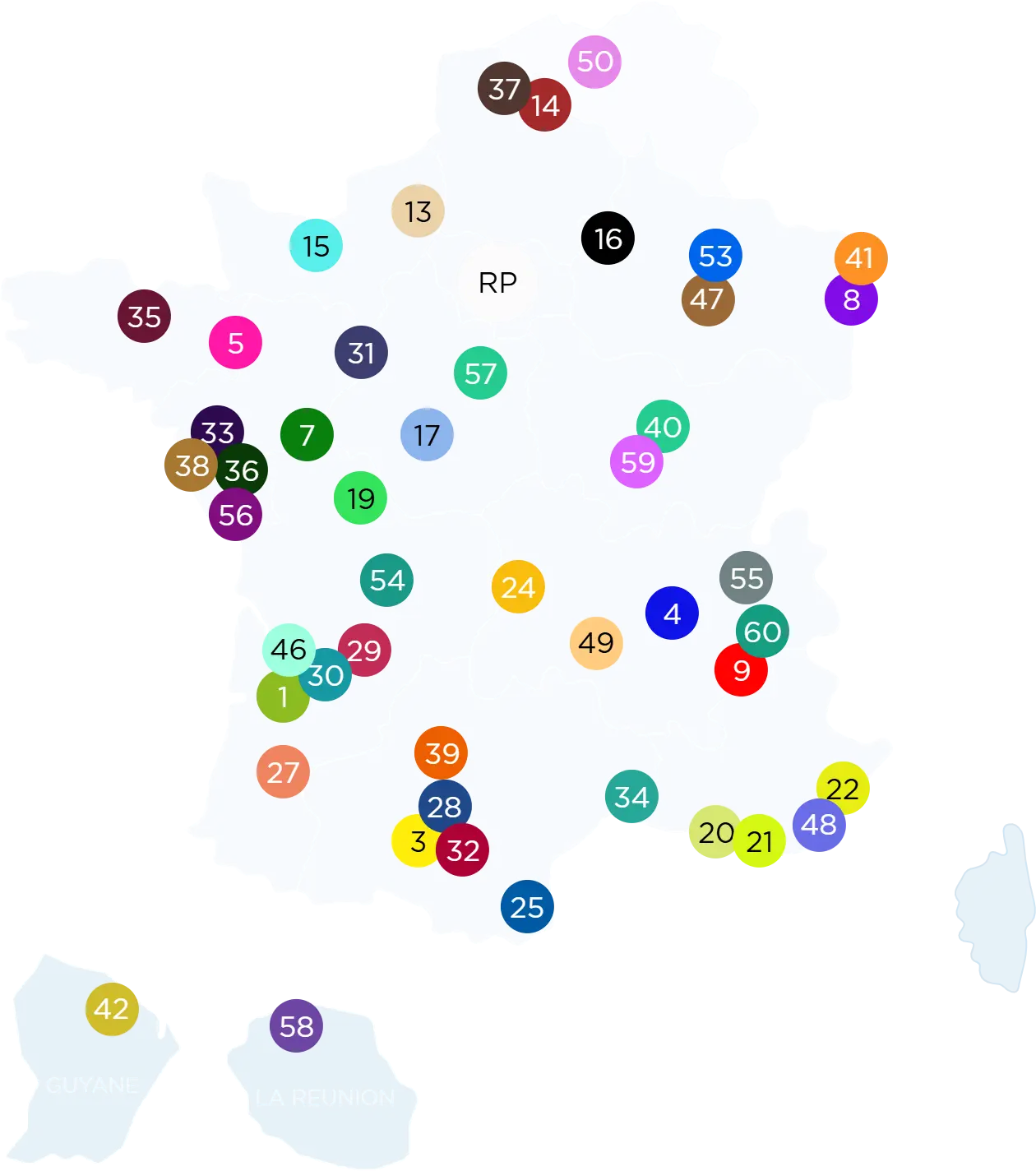
Mont de Marsan (APHP)
CHU Bordeaux
CH Libourne
Hôpital Privé Francheville
Clinique Tivoli
Clinique Tivoli
CHU Limoges
CHU Poitiers
CH Département de Vendée
CHU de Nantes
Clinique Nantes Atlantis
Clinique Santé Atlantique
CHU Angers
CHU Tours
CHU d’Orléans
Pôle Sud Santé-Le-Mans
CHU Rennes
Hôpital Privé des Côtes d’Armor
CHU Caen
CHU Rouen
Hôpital Privé La Louvière Lille
CHU Lille
Hôpital Universitaire de Bruxelles
CHU Reims
CHU Nancy
Institut Cancer de Lorraine
ICANS
CHU Strasbourg
CHU Dijon
CGFL Dijon
CHU Clermont-Ferrand
CH Annecy Genevois
CHU Lyon
CHU St-Etienne
Clinique Belledonne
CHU Grenoble
CHU Nice
Clinique Saint-George
APH Marseille
IPC Marseille
CHU Nîmes
Médipôle Cabestany
Clinique du Pont de Chaume
Clinique la Croix du Sud
Clinique Pasteur
CHU Toulouse
CHU Bellepierre à
Saint-Denis La Réunion
CH de Kourou Guyane
2006
UroCCR foundation
2011
INCa-certified clinic-biological database
2013
UroCCR national deployment
2016
INCa-dgos ambu-rein – designation
for ambulatory kidney surgery
2017
Rein-3d-print
2019
Uroconnect
co-developement
2021
National research
networks + carare
2022
Award-winning
project – DiPRU (BPI)
2022
Award-winning
project – Uropredict
2022
SNDS linkage with UroCCR-chain
2023
French National
Authority for
health certification
2024
ANR ”Clinical
Investigation Network”-certified
2025
Inclusion in the
health data hub catalogue
2025
IBISA-certified
Data
11,818,284
data
7,72
studies on
average / patient
17,923
patients included in
at least 1 study
30,53
months of
average follow-up


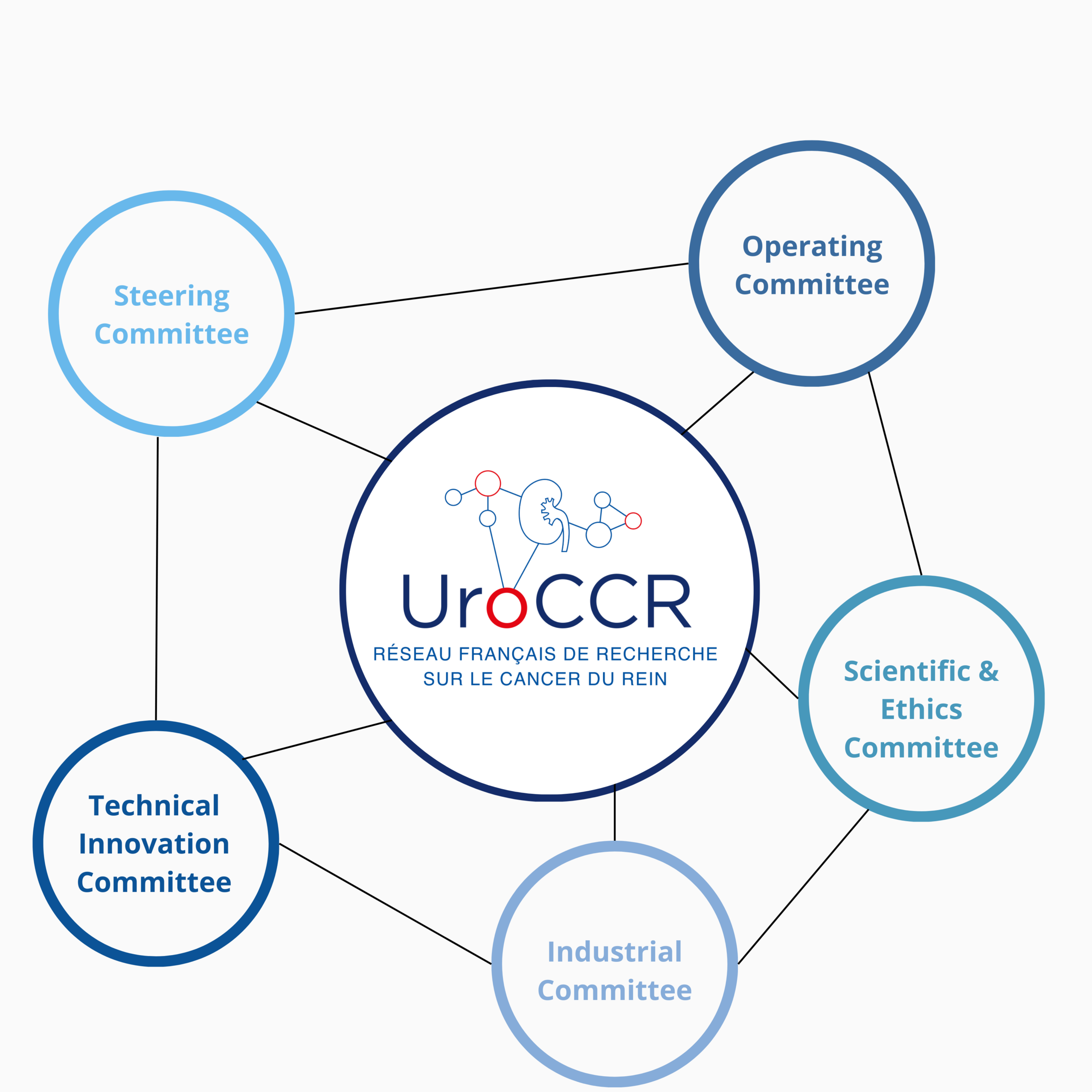
In order to achieve the objectives of the UroCCR project, governance is based on consultation and medico-scientific consensus. UroCCR is composed of five committees, represented below.

The Steering Committee is composed as follows:
It is composed as it follows:
The Operating Committee is composed as follows:
The Industrial Committee is composed as follows:
The Technical Innovation Committee is composed as it follows:
Retrospective projects on the UroCCR network follow a structured process to ensure data quality and regulatory compliance:
Project’s submission
Scientific and ethical evaluation
Definition of data and participating centres
Data extraction
Analysis and research results
Publication and valorisation
2
Kremlin-Bicêtre
6
HEGP
10
St-Joseph
11
Mondor
12
La Pitié Salpêtrière
18
Bichat
23
Tenon
26
Claude Galien
43
Hôpital Foch
44
CHU Cochin-Port Royal
45
CH de Fontainebleau
51
CH Sud Francilien
52
Institut Mutualiste Montsouris